
OVERVIEW OF THE CDSCO FOR MEDICAL DEVICE
CDSCO registration is a approval from the side of the Central Drugs Standard and Control Organization. The CDSCO is the national regulatory authority responsible for ensuring the safety, efficacy, and quality of medical devices and in vitro diagnostic (IVD) products in India.
Licenses can vary based on the class of device (Class I, II, or III) and the risk level associated with it.
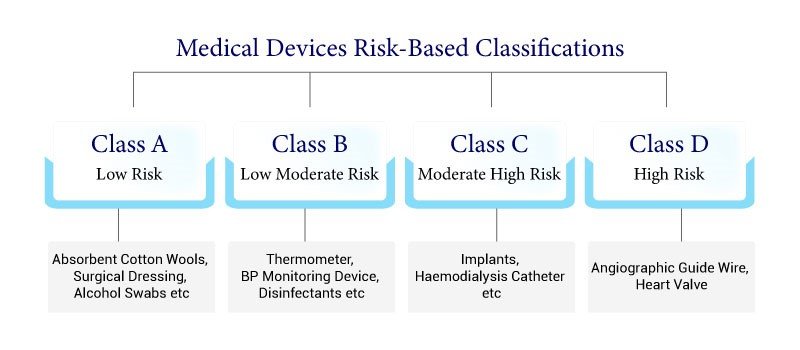
MEDICAL DEVICE RISK BASED CLASSIFICATION
Low Risk
Absobent Cootton wools,surgical
Dressing,Alcohol Swabs etc
Low Moderate Risk
Thermometer,BP Monitoring
Device,Disinfectants etc
Moderate High Risk
Implants,Haemodialysis
Catheter etc
High Risk
Angiographic Guide
Wire Heart Valve
Medical device licensing will be even more vital in the future as innovation accelerates and patient safety continues to be a priority. Staying up-to-date with regulatory changes and maintaining compliance will be essential for businesses to thrive in the global healthcare market.
CDSCO
-
Medical Device Import
-
Medical Device Manufacturing
-
medical Device Wholesale
-
Cosmetics Import
-
Drug Import
-
ADC NOC
-
Dual Use NOC
Our services for medical device units include:
- Manufacturing License (MD 5)
- Loan License (MD 6)
- Import License (MD 15)
- Test License for Import &
- Manufacturing (MD 13 & MD 17)
- Distribution License (MD 42)
- ISO 13485:2016 Certification & Implementation
- CE Marking & Consultancy
- Trademark Registration Support
- Import-Export Consultancy and Shipping Services

